Welcome to Hyfe Research
Introduction to the Hyfe CoughMonitor Suite (CMS)
The Hyfe CoughMonitor Suite (CMS) is a research tool used to identify and quantify cough in real world environments. It consists of three main components, (1) a CoughMonitor smartwatch (CoughMonitor), (2) a Companion app for smartphones, and (3) a web-based dashboard. The Hyfe CMS is a well-established cough monitoring technology that has been used globally as a research tool under IRB approval.
The cough monitoring software runs entirely on the CoughMonitor, no sounds are recorded, retained, or synced to the cloud, ensuring complete privacy. This guarantees no audio or conversations are recorded, the CoughMonitor only timestamps coughs in real time.
The timestamps of coughs are transmitted via Bluetooth automatically to the Companion app that runs on the study participant's smart phone (or provisional, if required). The Companion app also has the ability to record patient-reported outcomes (PROs), and send push reminders and alerts to the study participant. All information is transmitted securely to the cloud, and only cough timestamps are available to the research teams, as no audio is recorded or stored.
The dashboard is a secure web-based portal for study teams to access cohort-level data and cough monitoring insights (total cough count, cough rate, among other metrics), as well as monitor CoughMonitor charging behavior and wear/non-wear status (using the PPG sensor on the CoughMonitor watch). The CMS dashboard provides the most granular view of cough data, each cough is timestamped to millisecond precision with high accuracy enabling exploration of multiple derived metrics such as cough bouts, cough epochs (of any definition), cough-free time, and more.
Study participants are instructed to wear the CoughMonitor continuously during the day and to charge it by the bedside at night. The CoughMonitor has over 80 hours of battery life and is waterproof. It continues to record cough timestamps while charging, thus providing continuous 24-hour cough data throughout the entire study period. In prior studies, adherence to cough monitoring has been very high, for example, a recent ongoing phase 2b trial with over 130,000 hours of monitoring to date has shown over 96% adherence using Hyfe’s CoughMonitor Suite.
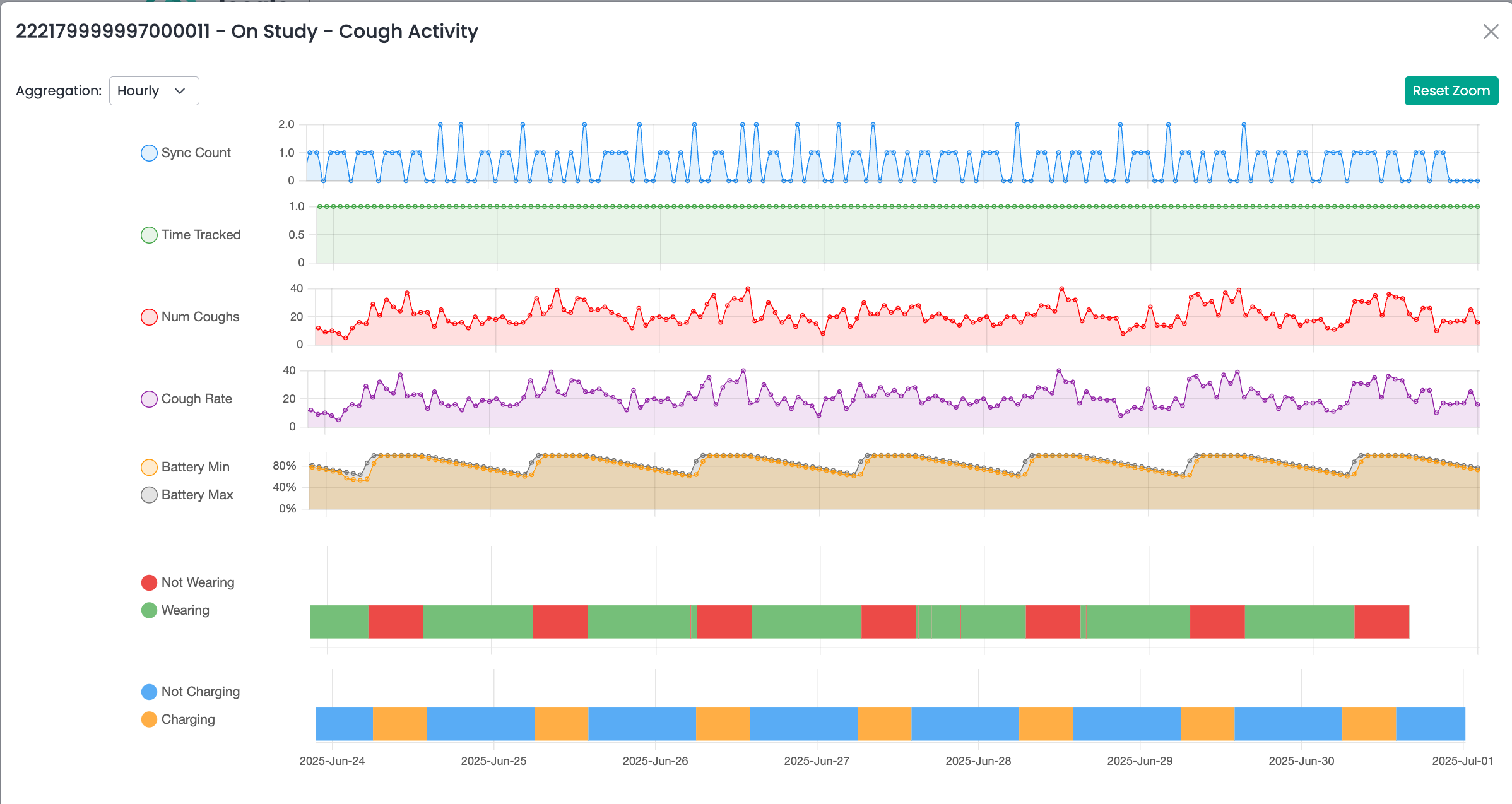
Hyfe’s Cough Insights for Research
Adherence:
We have high participant adherence and acceptability as recorded in the clinical trials using Hyfe’s CoughMonitor Suite. The thoughtful design of the CoughMonitor Suite and Hyfe’s strong support infrastructure have resulted in excellent adherence rates, for example, in a phase 2b trial, over 130,000 hours of monitoring were completed with >96% adherence over the period of a few months.
The Hyfe CoughMonitor Suite is built with long-term adherence in mind: the dedicated CoughMonitor is designed to be lightweight, slim, waterproof, and comfortable in a familiar form factor, encouraging daily use.
Interviews show that study participants prefer wearing an unobtrusive smartwatch (e.g., Hyfe’s CoughMonitor) for continuous cough monitoring rather than a pendant on their neck or an adhesive device on the chest.
Study participants and IRBs have had no reported privacy concerns about Hyfe’s CoughMonitor Suite, as the research tool does not record, store or sync audio to the cloud. The only data collected by the Hyfe CoughMonitor Suite is in the form of timestamps (of every cough, battery level, wear/non-wear status, etc.)
With up to 3 days of battery life and a simple magnetic charger the CoughMonitor is designed to be easy to use for study participants. All participants are instructed to wear the CoughMonitor during the day and charge it at their bedside overnight, where it will continue to monitor coughs while charging, resulting in 24/7 cough monitoring.
The CoughMonitor can store extended periods of data on-device in case syncing with the CoughMonitor Companion app is delayed.
Participant alerts, such as low battery warnings and wear/non-wear detection (using PPG sensors), are customizable per study protocol. Study teams benefit from visual, near real-time adherence tracking via the web-based dashboard, along with configurable email alerts and predefined actions.

Language for IRB Submissions
Hyfe has been IRB-approved in multiple studies around the world. Below we provide examples of content that may be useful as you build your own IRB proposal for studies involving Hyfe's CoughMonitor Suite.
Intended Use:
The CoughMonitor Suite consists of a dedicated smartwatch, a companion app and a web-based dashboard intended to be used by researchers in IRB approved clinical trials. The CoughMonitor quantifies cough data by passively detecting and time stamping each cough event. Cough data (timestamps) is uploaded to Hyfe’s Cloud via Bluetooth using a smartphone based app (CoughMonitor Companion app), and transmitted to researchers via a web-based research dashboard in near real time. This tool is designed for research trials that include cough frequency as an exploratory endpoint.
Study Protocol and Informed Consent Form:
You can use Hyfe’s standard protocol and the Consent Form to design your own study.
Privacy:
Hyfe takes user privacy extremely seriously. No audio is recorded, stored, or synced to the cloud. The CoughMonitor tracks sound level through the device’s microphone. The first “peak detection” algorithm identifies 0.5 second explosive sounds that are similar to a cough sound. Then a second “cough recognition” algorithm is used to classify these “explosive sounds” between coughs and non-coughs. All processing happens on-device, that is, no acoustic data ever leaves the watch. Only timestamps of the recognized cough sounds are sent to Hyfe’s Cloud.
Cough data is transmitted to researchers via a OTP (magic link sent to your email) protected research dashboard in near real time. Only approved study site members can access the web-based dashboard.
See Hyfe’s Privacy Policy for CoughMonitor Suite here.
Anonymization:
Hyfe provides research sites with unique email-password participant pairs that are only known to them, allowing researchers to be the only party capable of linking the cough data with the participants identity by means of the study ID number.
Data Storage:
Cough data (timestamps) are transmitted via an encrypted connection to Hyfe’s secure cloud. Data can be retrieved via a dashboard or API. The cough data is never linked to personal identifiers. Data can be deleted upon request.
Medical Decision Making:
CoughMonitor Suite is a research tool to be used in investigator initiated studies. It’s not cleared for the diagnosis or management of clinical care.
FAQs
What is the Hyfe CoughMonitor Suite?
The CoughMonitor Suite is Hyfe’s research product and consists of:
- A CoughMonitor, a smartwatch that counts coughs
- A CoughMonitor Companion App, a phone-based app available on iOS and Android
- A CoughMonitor Suite Dashboard, a web-based app for managing participants
What is CoughMonitor?
The CoughMonitor smartwatch is a watch running cough monitoring software, intended to be used by researchers in IRB-approved clinical trials. The CoughMonitor quantifies cough data by passively detecting and time stamping each cough event. Cough data (timestamps) is transmitted to Hyfe’s Cloud via Bluetooth using a phone based app named CoughMonitor Companion App.
Researchers can access cough data via a web-based dashboard in real time. This tool is designed for research trials that include objective cough frequency as an endpoint.
What data does the CoughMonitor Suite collect?
Hyfe provides a timestamp of each cough. This longitudinal continuous cough frequency data is accessible in near real time. In addition, Hyfe tracks whether the watch is being charged or not, and whether it is being worn or not (using the PPG sensor).
How accurate is the CoughMonitor Suite?
A clinical validation study demonstrated that the hourly coughs generated by the Hyfe system - when used in real-world conditions - correlated very closely with the hourly coughs counted by trained human annotators (Lin's Concordance Correlation Coefficient of 0.975). The same study showed a median sensitivity to cough-seconds of 90%, with fewer than 1.6 false positives per hour.
How can I use the data?
The use of the data depends on your research objective, though it is important that data collected by the CoughMonitor is only used observationally, never to drive clinical decision-making.
Hyfe´s cough-monitoring technologies have been employed in over 50 trials, and here are some of the different use cases for cough monitoring:
- Cough Science: To describe the natural history of cough in specific disease in order to gain fundamental disease insights and design better intervention trials
- Drug Development: To monitor changes in cough in drug development trials spanning proof of concept to regulatory enabling Phase III studies.
- Disease Management: For research designed to develop or test predictive algorithms that detect early stages of (or deterioration in) specific diseases.
Can Hyfe analyze the data for me?
Yes! Hyfe has a dedicated team that provides analysis services as needed. For example, our statisticians can use standard statistical approaches to determine feasibility (measured as adherence) acceptability (with a qualitative exit interview), cough rates, patterns, concordance of subjective and objective measures including clinical data and bouts (using various definitions). In addition, implications for future study design can be modeled on the impact of cough frequency and variability of sample size requirements for intervention studies.
Do I need to sign an agreement with Hyfe?
Yes. Hyfe signs agreements with all research partners. You can download Hyfe’s standard Data License Agreement and the Device Loan Agreement. Alternatively, you can propose using your institution’s templates.
Is the CoughMonitor Suite a medical device?
No, CoughMonitor Suite is not a medical device. It is a research tool used to measure cough frequency in studies. It is not intended for use in diagnosing, treating, or monitoring patients. It is not intended for clinical decision making and does not provide medical evaluations. The system is solely intended to assist in the collection of objective data to support scientific research.
How does Hyfe preserve patient privacy?
Hyfe takes user privacy extremely seriously. Hyfe monitors sound levels through the watch´s microphone. The first “peak detection” algorithm identifies explosive sounds that are similar to a cough sound. A second “cough recognition” algorithm classifies these sounds as either coughs or non-coughs. This classification process happens on the watch itself thus no acoustic data ever leaves the devices. Only timestamps of the recognized cough sounds are transmitted to the cloud.
Read the CoughMonitor Suite privacy policy here: https://www.hyfe.com/cms-privacy-policy
Does Hyfe require IRB approval to use the CoughMonitor Suite?
For academic or medical research, ethical approval is required, since any research on human subjects typically requires IRB approval.
What compliance standards does the CoughMonitor Suite follow?
Our internal data processes are HIPPA and GDPR-compliant, and in concordance with ISO 27001.
The CoughMonitor watch is a consumer device, with the relevant consumer certificates including a CE mark.
Does the CoughMonitor detect other people’s cough?
CoughMonitor is optimized for the best performance at a distance of up to 3ft (~90cm). CoughMonitor can detect other people’s coughs if it is in very close proximity to other coughing people. Based on our investigations, other people’s coughs captured in real world environments (e.g., as participants visit offices, take the metro, go grocery shopping, etc) is usually very minor and therefore not having an impact on one's longitudinal cough trend and pattern.
While this is generally not a concern for research trials, some studies have an exclusion criteria that excludes individuals who are living with other people who chronically cough.
Where is data stored?
Cough data (timestamps only) are transmitted via an encrypted connection to Hyfe’s secure cloud. Data can be retrieved via a web-based dashboard. The cough data is never linked to personal identifiers and can be deleted upon request.
Can I collect Patient Reported Outcomes (PRO) using the CoughMonitor Suite?
Yes, you can collect PROs (such as the NRS and other) using the CoughMonitor Companion App.
Are there any phone requirements for the CoughMonitor Companion App?
The phone app is compatible with the vast majority of smartphones in use. Specifically, all Android phones running version 12.0 and above, as well as iOS devices on version 16.0 and newer. Minimum requirements for the Bluetooth Version: v4.2.
Contact us for a Quote
Please note the cost is based on the number of devices your study requires and the duration (months) you want to keep them. This price includes:
- Smartwatch devices and onboarding site training
- License for Hyfe’s cough-monitoring software
- Access to Hyfe’s Research Dashboard
- Access to Helpdesk & Technical Support
- Trial strategy, project management and protocol development advisory
Analysis services are not included in this pricing. These costs vary depending on the type of analysis needed for the study. Hyfe can provide you with a customized quote including data analysis.
To get started on your quote, email Hyfe Chief Medical Officer peter@hyfe.com or fill out our quote form with any specific details you would like to share about your proposed study and someone from our team will be in touch.
Subscribe to Cough Science News - our curated monthly email newsletter - to stay in the loop on the latest in cough science, continuous cough monitoring insights, Q&As with our research partners, and upcoming relevant events.
